|
Press The Start Button
Have Speakers Turned On
Allow Large Files Time To Download If Not Using Broadband or DSL
To Best Enjoy Streaming Video and Sound, Configure Windows Program for:
Real Player, Quick Time, and Windows Media Player
Well, Folks! Dr "B" Is Reviewing Me In All Haste!
For:The Neutron Bomb!
This Is What He Is Reviewing Me On:Or, Should I Say...Teaching Me!
Ionization
This is the process whereby an electron is removed from an atom, molecule, or ion. In its final state, the transition from a neutral atom consisting of two parts; one is a positive ion and a free electron.
Radiation Monitoring
Of The U. S. Atomic Energy Commission / Division of Technical Information, 1971
When orbital electrons are freed from neutral atoms, this process is called Ionization.
Ionization results in two charged particles; the electron, which is negative. It is a free electron. The rest of the atom bears a positive charge.
It is often caused by radioactivity.
What Is Radioactivity And Radiation?
"Some atoms are called radioactive. A radioactive atom has an unstable nucleus, which it changes to a stable nucleus by actually ejecting a part of the nucleus. This action is sometimes accompanied by a release of electromagnetic energy. The ejected nuclear material and released energy are called radiation. The radioactive atoms are frequently referred to as contamination when they are encountered in undesirable locations.
"The three major types of radiation are alpha, beta, and gamma radiation. A brief description of each follows.
"Alpha radiation is a particulate radiation; that is, when alpha radiation occurs, two protons and two neutrons are ejected as a single particle from a radioactive nucleus.
"Beta radiation is also a particulate (has mass & weight) radiation, the beta particle being an electron emitted from a radioactive nucleus. ...the nucleus of an atom consists of neutrons and protons and that the electrons were in orbit around the nucleus. How, then, can a beta particle be an electron emitted from a nucleus? The answer is that a neutron in the unstable radioactive nucleus converts to a proton and an electron. The proton remains in the nucleus, but the electron is ejected.
"Gamma radiation is released energy, belonging to the same family of electromagnetic radiations as light, radio waves, ultraviolet radiations, etc. X rays are the same as gamma radiation except that they are produced differently.
"Once radiation is released from a radioactive nucleus, what happens to the ejected particles and energy? " We will show this graphically shortly.
- Radiation particles and energy do interact with and affect surrounding atoms. Just as a bullet will affect a board it strikes, radiation particles and energy will strike and perhaps change atoms in their path.
- The distance that radiation and energy will travel depends on the energy of the particle or gamma ray when it is emitted and on the frequency with which it interacts with the atoms along its path. For particulate radiation this frequency, as a rule of thumb, depends on the size of the radiation particule.
For example, the heavy alpha particle will usually not penetrate an ordinary sheet of paper, but to stop the much lighter beta particle will require a sheet of aluminum. Gamma radiation, having no mass, can be stopped only by thick sheets of lead or concrete.
"A fourth type of radiation involves the release of neutrons from atomic nuclei." Neutron radiation is discussed later.
Radiation EffectsFollowing Figures From Radiation MonitoringU.S. Atomic Energy Commission/Technical Information Center
"If radiation has no effect on the surrounding material, we might regard it merely as an interesting phenomenon. But radiation does affect the material exposed to it; this fact becomes very important when the exposed material is a person. We know that, if a person is exposed to very large quantities of radiation, sickness and even death can occur. For this reason, radiation exposure of personnel handling radiation materials must be held to safe limits.
"The problem of exposure of personnel to radiation is twofold. First there is the problem of external radiation, that is, radiation received from sources outside the body. External alpha radiation presents little hazard because the alpha particles usually cannot penetrate the outer layer of dead skin. Since the outer skin layer is composed of dead cells, the amount of radiation they receive is little cause for concern.
"Beta Radiation, on the other hand, can penetrate far enough to damage living tissue. The even more penetrating gamma radiation will reach such important organs as the blood–cell–producing bone marrow.
"Since the body cannot distinguish chemically between radioactive and nonradioactive isotopes of the same element, radioactive materials can be chemically incorporated into the body if they are swallowed or inhaled. The result is a problem of radiation from sources internal to the body. If this happens, alpha radiation becomes very important; internally deposited alpha–emitting radioisotopes can be a severe problem. Beta radiation is also frequently a more severe problem when the radiation source is internally deposited. Gamma radiation is, of course, a problem whether the source is external or internal."
With this background, let us return to Ionization:
"Alpha, beta, and gamma radiations are known as ionizing radiations. As previously mentioned, an atom is normally electrically neutral with an equal number of electrons and protons. However, if somehow an electron is separated from its orbit around the nucleus, a pair of charged particles results (a Pair of Charged Particles is not Pair Production): The free electron bearing a negative charge and the remainder of the atom bearing a net unit positive charge. Radiation is one mechanism which produces ionization.
"A moving alpha or beta particle is largely "absorbed" by the process of ionization when it collides with an orbital electron and imparts enough energy to the electron to allow it to overcome the attractive force of the nucleus. In this interaction an ion pair is formed.
"The impinging particle surrenders a portion of its energy to the orbital electron. After numerous collisions its energy is reduced to zero, and the particle is then considered absorbed. De–energized beta particles become free electrons, which are often absorbed by positive ions; the alpha particle frequently captures two free electrons to form a helium atom.
"The process of absorption of gamma rays is somewhat more complex. Gamma rays are absorbed by three chief processes:
- The Photoelectric Effect, in which the total energy of the gamma photon is absorbed by an orbital electron;
- The Compton Effect, in which only a portion of the energy is absorbed by an electron and a gamma photon is deflected with reduced energy; and, an electron leaves too.
- Pair Production, in which the gamma photon is annihilated to form an electron and a positron (a particle the size of an electron, but bearing a positive charge).
....These are demonstrated in Fig. 1.
Note: An Ion Pair Is Not Pair Production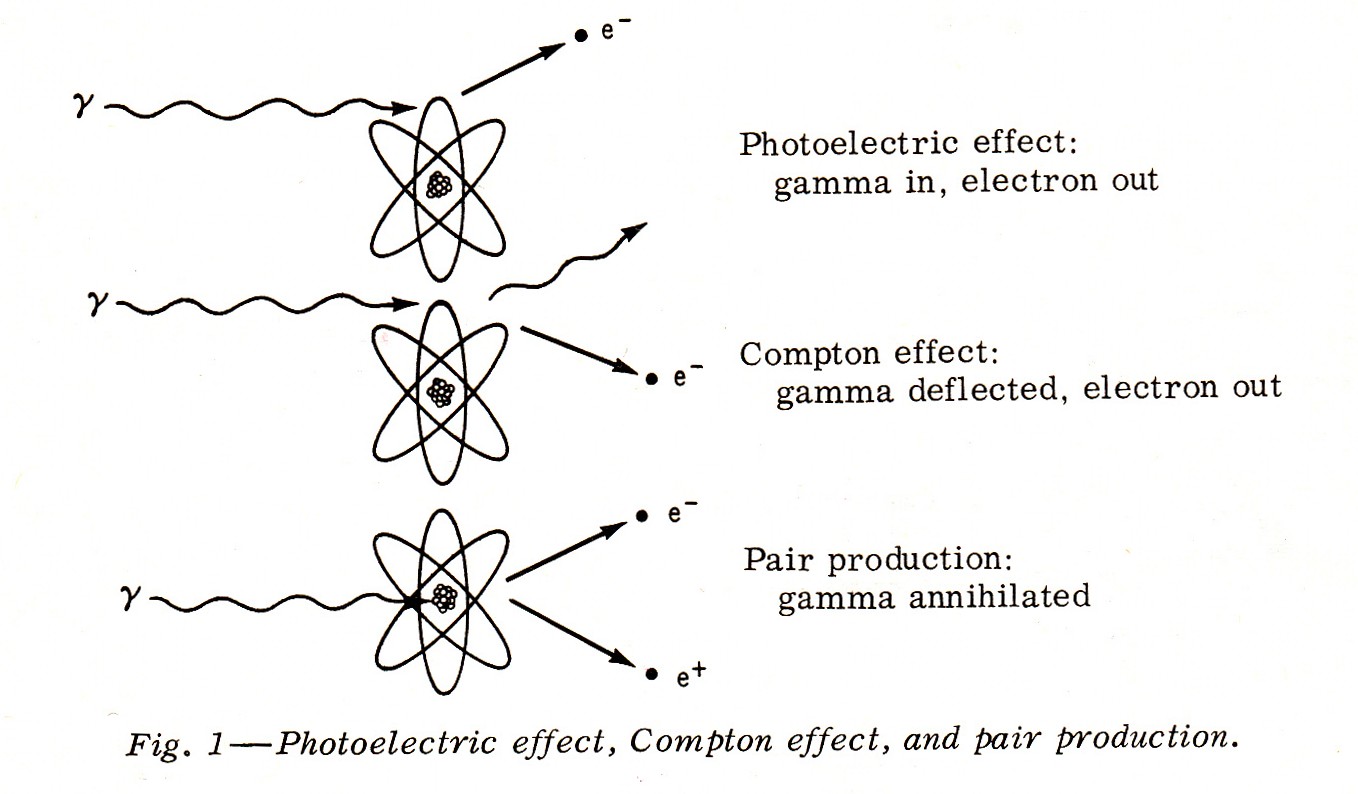 Keep in mind that an alpha particle can be stopped by a sheet of paper; a beta particle of the same energy with a thin sheet of aluminum; and only several inches of lead, or several feet of earth, or brick, or concrete will stop a gamma ray.
Keep in mind that an alpha particle can be stopped by a sheet of paper; a beta particle of the same energy with a thin sheet of aluminum; and only several inches of lead, or several feet of earth, or brick, or concrete will stop a gamma ray.
Neutrons
Neutron Radiation
"The neutron is one of the basic particles composing the atom and is found in the atomic nucleus along with the proton. Although the neutron is approximately the same size as the positive–charged proton, it carries no electric charge.
"When neutrons are emitted from the atomic nucleus, they act as ionizing radiation. Since they have no electric charge, however, neutrons do not ionize directly. Instead, they usually "strike" and interact with atomic nuclei; this interaction produces charged particles, which then cause ionization.
"Unlike alpha or beta particles, neutrons are not usually emitted spontaneously from the atomic nucleus, but they require some outside force to cause their removal from the nucleus.
"Another very important source of neutrons is atomic fission. Fission occurs with some isotopes of the heavier elements, notably uranium, thorium, and plutonium. When an atom fissions, the nucleus splits into two parts. Since each of these parts is a cluster of neutrons and p;rotons, they form the nuclei of new atoms called fission products. In addition, several neutrons and energy are also released. The fission products are radioactive and decay by emitting alpha, beta, and gamma radiations.
"Although spontaneous fission occurs, fission is usually caused when a neutron from some outside source strikes the fissionable nucleus. Neutrons are thus used to produce still more neutrons. Since neutrons produced by fission can cause additional fissions (if fissionable nuclei are available), which in turn produce more neutrons, it is possible to start and maintain a chain reaction. It is the chain reaction, of course, that makes the nuclear reactor possible," and we might add, the nuclear bomb!
Interactions With Matter
"Since the neutron carries no electric charge, it does not normally ionize by interacting with the orbital electrons of an atom as alpha or beta particles do. Instead, the neutron will interact with the atomic nucleus. Ionization does result, however, through secondary means, as many of the neutron–nucleus reactions produce directly ionizing radiations (alpha, beta, gamma, recoil protons)."
We have these following reactions that will occur with neutrons. We must be prepared if we build a shelter, as you will see later, because if we stop the neutrons, they can and will cause Secondary Reactions that must be controlled further in the brick, soil, or concrete, as the neutrons may have been controlled; but, then, we must control the secondaries with at least a foot or more of wet soil, concrete, clay & brick. For now, understand the following:
Inelastic Scattering occurs with neutrons with energies of about 10 Mev down to 1 Mev (1 electron volt 'ev' = The Amount of Energy Acquired By An Electron When It Moves Through A Potential of 1 volt). When inelastic scattering occurs, a nucleus absorbs the neutron and recoils. Then, a slower neutron (either the original incoming neutron or another one from the nucleus) is emitted along with gamma radiation: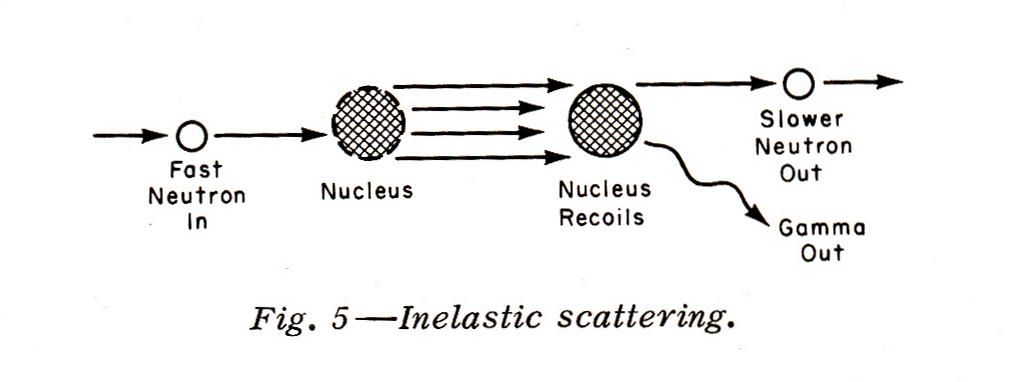
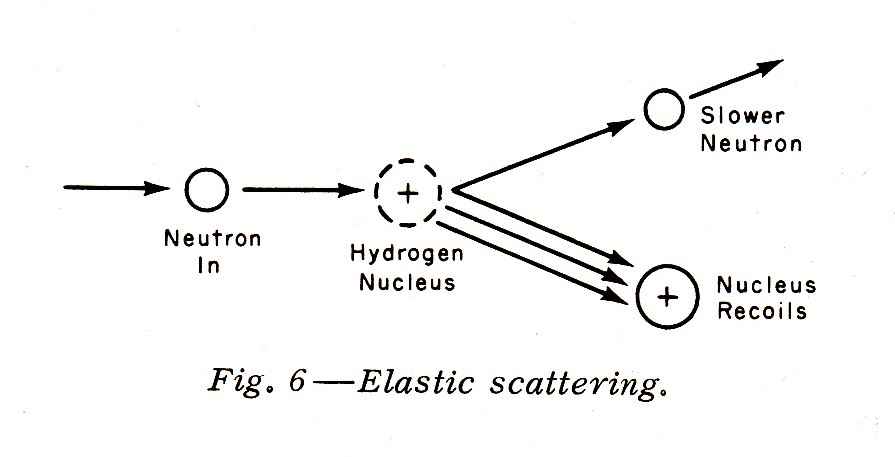
"Slower neutrons with energies of 1 Mev down to about 0.01 Mev (or 10 kev) interact by elastic scattering, particular with very light nuclei such as the hydrogen nucleus. Elastic scattering is similar to a collision of two billiard balls; the neutron loses some—or even all—of its energy to the hydrogen nucleus, which then recoils. The recoiling proton (remember that the normal hydrogen nucleus is a single proton), being a charged particle, is heavily ionizing. Since the human body contains a considerable quantity of hydrogen, elastic scattering and the ionization caused by the recoil proton are important biological considerations. Elastic scattering is demonstrated in Fig. 6."
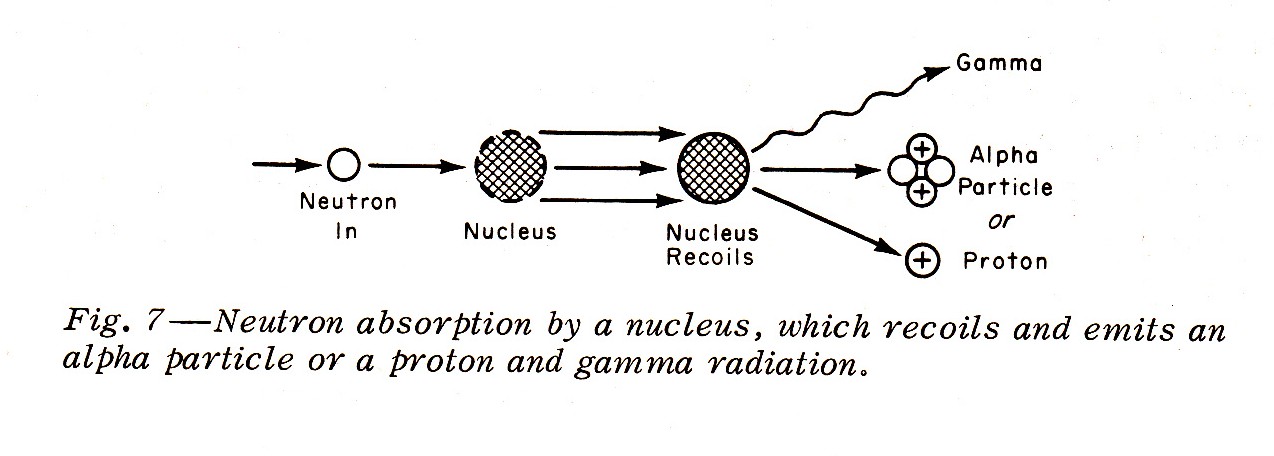
"Slower neutrons with energies generally less than 0.01 Mev may be absorbed by a nucleus with the nucleus recoiling and emitting an alpha article or a proton accompanied by gamma radiation (Fig. 7):"
"Such slower neutrons may also undergo radiative capture by a hydrogen nucleus to form a deuterium nucleus (deuterium is an isotope of hydrogen containing one proton and one neutron in the nucleus) with a gamma emission (Fig. 8):"
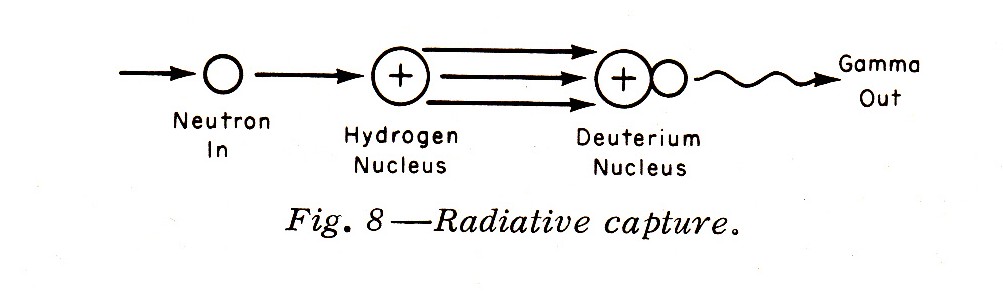 Note: Fission is an important interaction between neutrons and the heavier nuclei. Fission is usually produced by (but not only by) thermal neutrons, which have an energy of about 0.025 ev.
Note: Fission is an important interaction between neutrons and the heavier nuclei. Fission is usually produced by (but not only by) thermal neutrons, which have an energy of about 0.025 ev.
With this information, the definition of a Neutron Bomb is a small thermoneuclear weapon (fusion bomb—hydrogen bomb, design to release large amounts of neutrons to cause injury to humans and biota, but less damage to objects of a physical nature.
It is designed to cause damage to troops in the field; but, not to their tanks and the like. However, the small blast wave covers, depending on the size of the neutron bomb, out to about 300 or so yards. Hence, if one is within that radius, that person will definitely be injured or destroyed. The killing power of the neutron bomb is the neutron radiation damage!
However, in The Truth About The Neutron Bomb, The Inventor of the Bomb Speaks Out, by Sam Cohen. He writes:
Is this the capitalist bomb that doesn't destroy property? Is this the heralded weapon that kills people and leaves buildings intact? The answer is no, and survivors of the Nagasaki bombing would find such questions ludicrous. But this aspect never appeared in the media, because few people studied the Chairman's report.
Everyone was having apoplectic fits about a neutron bomb that does not exist.
What does exist is a bomb that destroys everything—not just the lives of enemy soldiers—and which isn't significantly different from the bombs it was supposed to replace. Isn't this the real issue?
I cannot see why so much fuss was made over the news that we were going to produce a "neutron bomb." The real neutron bomb was never more than a myth.
With This Background, Let Us Go Into This Deeper
In The Next Issue!You Will Learn What To Do For Neutrons of Any Blast Generating Them!
Dr. "B" Is Looking For Great Changes To Come Upon The Earth! He's Planning For It As Best He Can.
Ice & Flood Products Will Be Everywhere...When The Pole Shifts!
Dr. "B" Is Studying Intensively About Wild Fires &Their Influence On One's Moving About!
Dr. "B" Is Also Finding Out About The Types of FuelsThat Drive Wild Fires & Why They Do It! "Fire," He Says, "Will Be Everywhere When The Earth Starts Its Final Travail!
If You Did Not Get A Compass With Clinometer; Then, Here Is A Slope Meter! Print It Out & Follow Instructions!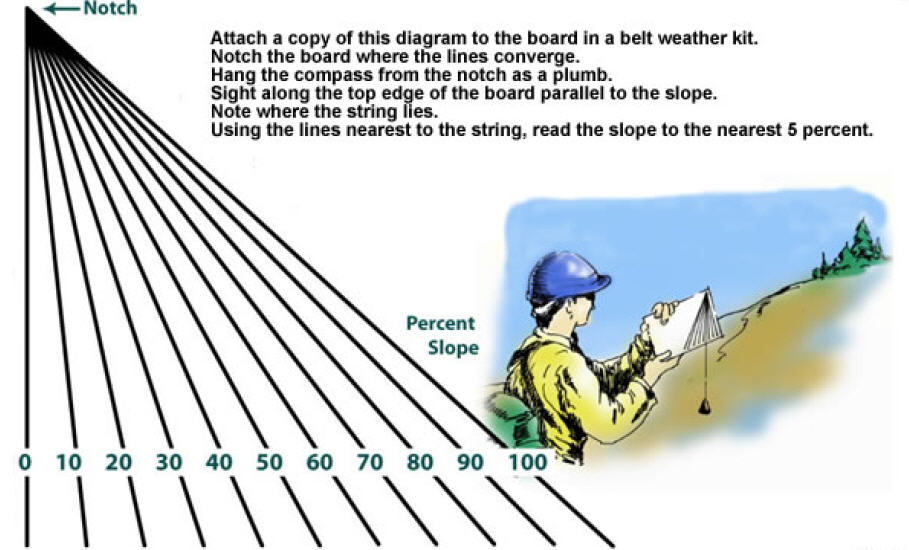
With The Coming Earth Changes...You Must Know Slope! Stay Off Slopes With 25 To 45 Degree Slants! This Is Where Most Avalanches Occur...Especially, With Snow & Ice!
Note:
"Proper cleansing was important, the Air Force noted (from the Bomb Tests from 1940s through the 1970s), because 'grease spots collected more than one hundred times as much contamination in passage through a radioactive cloud as a clean surface of equal area.'"
As a Worsening Crisis develops, stop wearing oily, unctuous perfumes; nor should one have braids, long hair, and pomades and grease in their hair. The radiation cloud that drops its debris; or, passes through an area, will leave more than one hundred times as much comtamination on you and your hair if skin and hair are greasy, than if the hair is cropped short and non–oily. This goes also for the skin with unctuous perfumes on it and oily lotions.
... To Be Continued ...
|
In accordance with Title 17 U.S.C. Section 107, any copyrighted work in this message is distributed under fair use without profit or payment for non-profit research and educational purposes only. [Reference:Cornell Law School]
In An UpComing Issue:
Something You Need To Know For What's Coming
Kong Sez:
ChemicalBiological.net uses Graphics, Videos, Audios, and other devices to communicate facts. As time nears to in America and the world, ChemBio Updates will be sent out several times a week, either in e-mail format or as a hot link to its WebSite, as information warrants.
As you use your computer, overtime, it slows down!
If your computer downloads slowly, you need to daily do the Following:
- Defrag your machine.
- Use a Cleaner, such as CCleaner (one can also use their Defragger) to Optimize your computer for better performance. Get them here: http://www.ccleaner.com/. It's Free!
- If you find a download from email coming down very slowly; simply close your computer and reboot. Then restart the download.
- Find out from your ISP how much file storage you have, you need at least 20 MB. Also, go there and clean up used files. The ISP does this for you every 30 or so days. If you receive large files, the ISP may bump them back because "no room at the inn."
Remember!
You Must Defrag Your Computer Regularly
Clean The Registry and Optimize the Machine Regularly
Or
It Will Run Very Erratic and Quite Slowly!
|
To Unsubscribe From Free ChemicalBiological WarFare Updates, Click Here: Unsubscribe
|
Register For Free Updates Here
|
|




 Note: Fission is an important interaction between neutrons and the heavier nuclei. Fission is usually produced by (but not only by) thermal neutrons, which have an energy of about 0.025 ev.
Note: Fission is an important interaction between neutrons and the heavier nuclei. Fission is usually produced by (but not only by) thermal neutrons, which have an energy of about 0.025 ev.



